Engineering Electronic Communication
Cellular-electrical connections would enable devices to combine specialties of the living world and the non-living technological world. This new class of smart, self-renewing nanostructured systems has the potential to revolutionize environmental sensing and energy harvesting applications and to open new avenues to program cellular behavior. Building towards this vision, the long-term objectives of the our research are to develop understanding of the principles which govern electron flow across living-non-living interfaces and to use those principles to create electron transfer pathways between individual living microbial cells and non-living electrodes. Our overall strategy is both to explore how naturally-occurring microbes achieve electron transfer to inorganic surfaces and to use genetic and materials surface engineering to create new ‘domesticated’ hybrid cell-electrode systems.
References and links:
- “Transforming exoelectrogens for biotechnology using synthetic biology.” M. A. TerAvest and C. M. Ajo-Franklin, Biotechnology & Bioengineering, 113, 687–697 (2016).
- “Crossing Over: Nanostructures that Move Electrons and Ions Across Cellular Membranes” C. M. Ajo-Franklin, and A. Noy, Advanced Materials, 27, 5797-5804 (2015).
- “CymA and exogenous flavins improve extracellular electron transfer and couple it to cell growth in Mtr-expressing Escherichia coli.” H. M. Jensen, M. A. TerAvest, M. K. Kokish and C. M. Ajo-Franklin, ACS Synthetic Biology, in press (2016).
- “The Mtr pathway of Shewanella oneidensis MR-1 couples substrate utilization to current production in Escherichia coli.” M. A. TerAvest, T. J. Zajdel, and C. M. Ajo-Franklin, ChemElectroChem 1, 1874-1879(2014).
- “Engineering of a synthetic electron conduit in living cells.” H. M. Jensen, A. E. Albers, K. Malley, Y. Y. Londer, B. E. Cohen, B. A. Helms, P. Weigele, J. T. Groves, C. M. Ajo-Franklin, Proc. Natl. Acad. Sci. USA,107, 19213-19218 (2010).
- C&EN Feature: “Building Better Bacteria.”
Assembly & Applications of S-layer proteins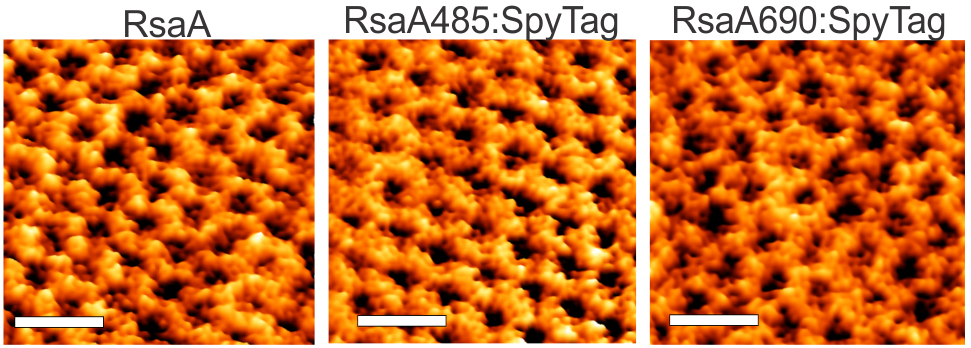 S (`surface’)-layer proteins form crystalline lattices on the outsides of many bacteria and archaea. These nearly ubquitous structures play a number of crucial biological roles: they serve as structural scaffolds, effect selective transport of ions and proteins, serve as templates for mineralization, and protect against phagocytosis. While the lattice structures of many S-layers are known, the dynamical mechanisms through which they form are poorly understood. A molecular-level picture of the assembly of the S-layer protein SbpA from Lysinibacillus sphaericus on lipid bilayers was obtained only recently by using in situ atomic force microscopy. AFM images elucidated the phase transition of amorphous precursors into the crystalline clusters composed of folded tetramers and subsequent growth without disappearance of any crystal clusters. Such a system demonstrates the non-classical crystallization behaviors in the S-layer assembly. Molecular dynamic simulations of the S-layer assembly using a coarse grain model also supported the non-classical nucleation arising from a dense liquid precursor, and subsequent growth of the crystal2. The long-term objectives of our research are to develop predictive understanding of S-layer protein nucleation and growth, so as to advance basic knowledge of the dynamics of coupled nanoscale phase separation and self-assembly and to enable control of the nanoscale structures. Together with the Whitelam, DeYoreo, and Bertozzi groups, we are using a combination of fluorescence microscopy, atomic force microscopy, and computer modeling to reveal the mechanisms underlying S-layer crystallization and to identify strategies for its control.
S (`surface’)-layer proteins form crystalline lattices on the outsides of many bacteria and archaea. These nearly ubquitous structures play a number of crucial biological roles: they serve as structural scaffolds, effect selective transport of ions and proteins, serve as templates for mineralization, and protect against phagocytosis. While the lattice structures of many S-layers are known, the dynamical mechanisms through which they form are poorly understood. A molecular-level picture of the assembly of the S-layer protein SbpA from Lysinibacillus sphaericus on lipid bilayers was obtained only recently by using in situ atomic force microscopy. AFM images elucidated the phase transition of amorphous precursors into the crystalline clusters composed of folded tetramers and subsequent growth without disappearance of any crystal clusters. Such a system demonstrates the non-classical crystallization behaviors in the S-layer assembly. Molecular dynamic simulations of the S-layer assembly using a coarse grain model also supported the non-classical nucleation arising from a dense liquid precursor, and subsequent growth of the crystal2. The long-term objectives of our research are to develop predictive understanding of S-layer protein nucleation and growth, so as to advance basic knowledge of the dynamics of coupled nanoscale phase separation and self-assembly and to enable control of the nanoscale structures. Together with the Whitelam, DeYoreo, and Bertozzi groups, we are using a combination of fluorescence microscopy, atomic force microscopy, and computer modeling to reveal the mechanisms underlying S-layer crystallization and to identify strategies for its control.
References
- “Engineering the S-layer of Caulobacter crescentus as a Foundation for Stable, High-Density, 2D Living Materials,” M. Charrier, D. Li, V. Mann, L. Yun, S. Jani, B. Rad, B. Cohen, P. Ashby, K. Ryan, & C. M. Ajo-Franklin, ACS Synthetic Biology, 8, 181–190 (2019).
- “Ion-specific control of the self-assembly dynamics of a nanostructured protein lattice.” B. Rad, T. K. Haxton, A. Shon, S.-H. Shin, S. Whitelam, and C. M. Ajo-Franklin, ACS Nano, 9, 180–190 (2015).
- “Recovery of critical metals using biometallurgy.” W.-Q. Zhuang, J. P Fitts, C. M. Ajo-Franklin, S. Maes, L. Alvarez-Cohen, and T. Hennebel, Current Opinion in Biotechnology, 33, 327–335 (2015).
